Silicon Carbide Production Process
While alternative production methods have emerged for selected high purity silicon carbide over the last years, the majority of SiC used today is produced using the so called Acheson process.
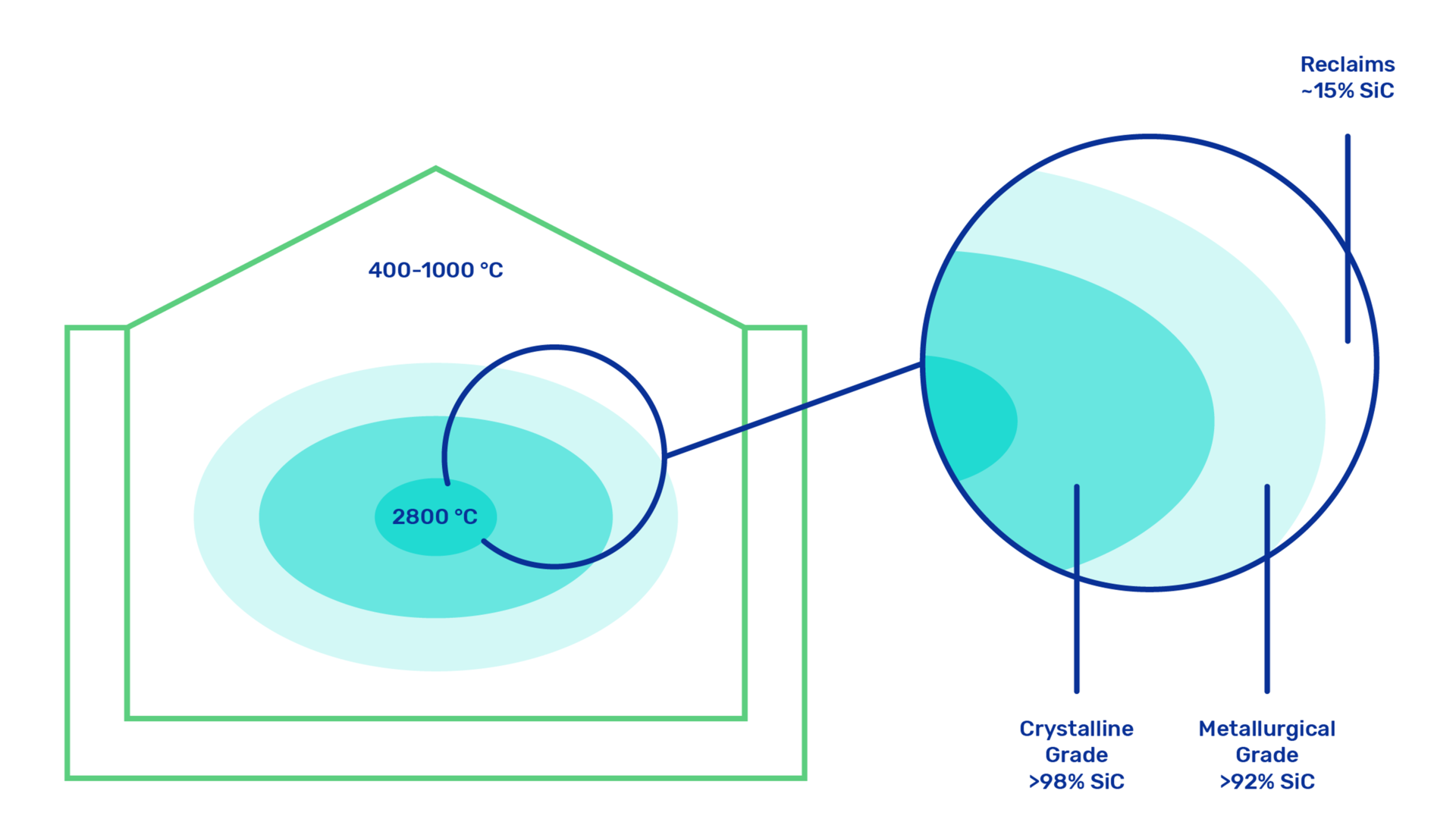
Silicon carbide (SiC) is a synthetic mineral most commonly produced in electrical resistance furnaces, by the Acheson process, named after the American E.G. Acheson who invented it in 1891. In an Acheson furnace, a mixture of carbon material (usually petroleum coke) and a silica or quartz sand is reacted chemically at high temperatures in the range of 1700 – 2500°C resulting in the formation of α-SiC following the main reaction:
The energy for the reaction is provided by the resistive heating of a graphite core done by connecting this core to two electrodes at both ends of the furnace.
SiC develops as a solid cylindrical ingot around the core, with radial layers ranging from graphite in the inside, to α-SiC (the highest grade material with coarse crystalline structure), β-SiC, metallurgical grade and finally un-reacted material on the outside. SiC can be produced as either black or green, depending on the quality of the raw materials.
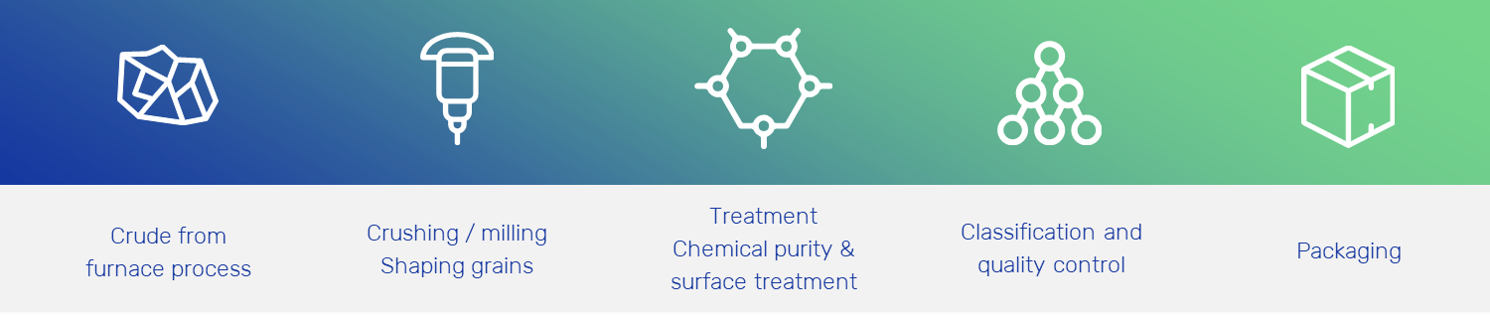
After a cooling period, the SiC ingot is sorted accurately and further processed for different applications. The SiC crude material is carefully crushed, classified, sometimes milled again, and optionally chemically treated in order to obtain the specific properties for which it will be applied.
Properties of Silicon Carbide
SiC is a ceramic material with an outstanding hardness, only surpassed by diamond, cubic boron nitride and boron carbide. The material is highly wear resistant and chemically inert to all alkalies and acids. It is also highly heat resistant. These properties make silicon carbide an outstanding abrasive and ceramic material to be used under extreme operating conditions.
- Density: 3.21 g/cm³
- Vickers hardness: 29 GPa
- Coefficient of Thermal expansion: 5·10-6/K
- Thermal conductivity: 50 to 100 W/m K
- Typical temperature resistance: 1500°C in air, 2400°C in inert atmosphere
- Specific heat: 750 J/kg K